Workbook for preparing a technical dossier for cosmetic ingredients
A cosmetic ingredient dossier organises the data and other information about your ingredient so you can prepare your offer to buyers in the cosmetic ingredient sector. It contains data sheets and other technical information that you need to promote your ingredient in Europe and meet European market requirements. This workbook contains information on what to include in a dossier and how to prepare it, including the technical data sheet (TDS) or specification, the safety data sheet (SDS), certificates of analysis (CoA) and a product data sheet (PDS) or sell sheet. The workbook also includes key references regarding information about social responsibility and how to meet buyers’ expectations.
Contents of this page
- Why do you need to have a dossier?
- Setting up your dossier
- Determining the INCI name for your ingredient
- Preparing the Technical Data Sheet (TDS)
- Preparing the Certificate of Analysis (CoA)
- Preparing the Certificate of Conformity (CoC)
- Preparing the Safety Data Sheet (SDS)
- Preparing the Product Data Sheet (PDS)
- Sampling procedures
- Corporate Social Responsibility
1. Why do you need to have a dossier?
The cosmetic ingredient dossier, electronic or in print, is the central reference of information about your ingredient, both from your own knowledge as well as from secondary sources. In the eyes of a buyer, you are the expert on what you are offering. The dossier becomes the source of information from which you become that expert. The dossier has to respond to the needs of the cosmetics industry and what they are looking for in their cosmetic ingredients.
All cosmetic ingredients have to meet the standard legal and market requirements of safety and efficacy, as well industry expectations in relation to price, volume and reliability of supply.
In addition to these requirements, cosmetic brands are also looking for the following set of desirable characteristics:
Natural
The industry is constantly looking for more natural ingredients, also commonly referred to as ingredients of natural origin.
Innovative
The cosmetics industry is always looking for innovative ingredients that meet the requirements imposed on all other cosmetic ingredients.
Origin
The cosmetics industry is looking for ingredients with an interesting or different origin. This includes not just the geographic origin, but also traditions of use, local benefits and production methods, among other things.
Social responsibility
Social responsibility is one of the main concerns of the cosmetics industry, after safety and efficacy. Consumers expect high standards of social responsibility regarding care for people and the planet. It is not only a business-to-business activity: social responsibility starts with traceability in the supply chain, but extends to ensuring that at least the minimum norms of social responsibility are respected throughout the supply chain. This may or may not involve certification, depending on the expectations of a particular customer.
The cosmetic ingredient dossier is the source of all the data and information needed to prepare the technical documents and promotional materials that relate to the above requirements and expectations of industry.
Elements of a cosmetic ingredient dossier
You can structure the document by dividing it in chapters on safety, efficacy, CSR and other legal and market requirements, using the information and data described in Table 1 below. The content of each chapter should enable you to provide information and data on the topics of each chapter as follows:
Table 1: Elements of a cosmetic ingredient dossier
| Efficacy | Corporate Social Responsibility (CSR) | Other legal and market requirements | |
| Evidence of safety | Basis for claims: robustness, statistical significance | Compliance with Nagoya protocol | Quality standards, such as Good Manufacturing Practice (GMP), Hazard Analysis and Critical Control Points (HACCP) and ISO9001 (International Organization for Standardization) |
| Risk mitigation steps | Depends on the type of ingredient: vegetable oil, essential oil, other plant extracts or derivatives | Compliance to generally accepted international standards of social responsibility | Volumes of raw material availability (basis and justification for this claim) |
| Maximum recommendation concentration | Prior experience of use in cosmetics industry | CSR standards and certifications: third party audits | Processing operations used |
| Full understanding of the composition and assurance of the botanical identification | Knowledge of traditional use | Use of Supplier Ethical Data Exchange (SEDEX) and other CSR platforms | Processing capacities: Volumes available per year |
| Access to technical services: local, international, which tests, by whom | Bibliography | Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) | |
| Depends on the type of ingredient: vegetable oil, essential oil, other plant extracts or derivatives | Traceability | ||
| Prior experience of use in cosmetics industry | Promotional materials (sell sheet), including photographs and video | ||
| Knowledge of traditional use | Seasonality | ||
| Technical documents: specification, safety data sheet, certificates of analysis; declarations of no animal testing, no allergens, no genetically modified organisms (GMO) | Shipping options | ||
| Bibliography | Packing options |
For this guide we are going to focus on the technical documentation and aspects of corporate social responsibility.
The importance of technical documentation
The cosmetics industry is a branch of the chemical industry. European cosmetic industry legislation is based on a risk management approach. This means there must be a good understanding of the maximum levels of chemical components that can be used safely in cosmetics, including a proper margin of safety. The ingredients dossier contains the raw data and information to make decisions on safe use of the ingredient in a cosmetic product.
For example, essential oils are powerful chemicals and generally must not be used directly on the skin. The industry and its regulators investigate the maximum safe concentrations in cosmetic ingredients and their individual components and ensure adherence to these maximum levels, either through legislation or industry codes of practice.
The dossier for an essential oil contains the data relevant to the determination of maximum safe concentrations in actual use. The dossier must include a description of the full chemical composition of your ingredient, including any impurities. Understanding the chemical composition of a cosmetic ingredient is a fundamental principal of the industry. Without a clear understanding of its full chemical composition, it is not possible to make a conclusive statement on the safety of that ingredient. If the full composition is not known, two main risks remain:
- that certain dangerous components may have not been tested;
- that levels of dangerous components in the ingredient have not been adequately controlled through the production process.
It is important to note that the European cosmetic industry maintains a high level of vigilance regarding the quality and safety of cosmetic products. Requiring well-organised ingredient dossiers is part of ensuring such high standards of safety throughout the industry. Incidents from using a cosmetic product and its components can be quickly brought to the attention of the authorities, so they can initiate investigations. With a population of approximately 500 million people using a wide range of products on a daily basis, significant concerns regarding cosmetic ingredients can be detected relatively quickly, and often statistical significance can be established.
Cosmetic ingredients have been categorised into three groups for the purposes of this workbook:
- vegetable oils
- essential oils
- botanical extracts
Underlying the information presented in this dossier is that companies comply with a minimum standard of quality management. At the heart of a quality system is the hazard analysis and critical control point (HACCP) methodology. Although originating from the food industry, the principles of HACCP apply as minimum requirements to processors of cosmetic ingredients. As an industry expectation, all suppliers of cosmetic ingredients need to be operating with HACCP, ideally certified HACCP. In addition to HACCP, the cosmetic industry also requires compliance with the cosmetic ingredient good manufacturing practice (GMP) standard published by the European Federation for Cosmetic Ingredients (EFFCI), which is based on the ISO9001 standard. If you are not implementing HACCP in your company then it is highly recommended that you find out how to do that.
Vegetable oils
All vegetable oils have a similar chemical structure. They are comprised of triglycerides or fatty acids. Different oils have different compositions with regard to their fatty acid profiles. Different profiles perform differently in cosmetics. Hence the fatty acid profile is one of the most important parameters to include in your dossier.
Most pure vegetable oils are inherently safe ingredients when used in cosmetics. Some vegetable oils contain naturally occurring toxic fatty acids, such as erucic acid in rapeseed. In these cases, the toxic fatty acids must be removed prior to human and animal consumption or use in cosmetics, unless the risk is adequately managed.
When vegetable oils are used in cosmetics, the most common problems involve impurities. These impurities are typically proteins found in the oilseed, which remain in the oil due to lower quality processing. Proteins can cause allergic reactions in humans. For some consumers, these allergic reactions can be very serious and even life-threatening. Therefore, European manufacturers typically expect very low levels of protein in oils. Suppliers selling directly to manufacturers will be expected to include an allergen declaration that states the level of protein in each batch.
Essential oils
Essential oils are natural plant extracts. They can contain hundreds of different chemicals. These chemicals are known for their fragrance properties, but also for their anti-bacterial properties and their effects on mood and emotions. Essential oils are powerful chemicals. Some of them can cause very serious health problems. Others can cause adverse skin reactions. Essential oils are more easily flammable than vegetable oils.
Considering these extra hazards, legislators and the cosmetic industry have thoroughly studied essential oils to determine safety levels for their use in cosmetics, as well as the precautions that need to be taken when transporting and handling such powerful chemicals.
Botanical extracts
Botanical extracts typically contain the active components that are used in cosmetics to be able to make claims such as anti-ageing, reduction of wrinkles, hydrating skin and soothing sensitive skin. Botanical extracts require safety and efficacy data.
- Generally speaking, botanical extracts are safe. They are naturally derived and extracted using known solvents. Plants, however, provide not only ingredients for many medicines, but also some of the most potent poisons. Consequently, botanical extracts require validated, reliable safety data. The short- and long-term toxicity risks must be known, so product safety assessors can determine safe levels for use.
- Investing in expensive safety testing can of course be justified more easily when there is good evidence of the cosmetic benefits that a specific concentration in a cosmetic provides over a time period.
What must your dossier contain?
Raw data for all of the above needs to be filed in an ingredient dossier. The data will also be used to prepare the typical technical, safety and promotional documents expected by the industry. As a supplier of cosmetic ingredients, you need to have this dossier ready to help prepare your commercial offer. The dossier itself is a confidential document to be used selectively and carefully with your customers.
The three most important documents in the dossier are:
- Technical Data Sheet (TDS)
- Safety Data Sheet (SDS)
- Product Data Sheet (PDS)
In addition to these data sheets, other supplementary technical information required includes:
- Certificates of Analysis (CoA) of a batch
- certificates of conformity
- allergen declarations
By the time you have prepared the above data sheets and offer your cosmetic ingredient to a European company, you already should have scientifically validated data on the safety and efficacy of the cosmetic ingredient you supply. This data is either derived from your own investigation or it is already in the public domain. If the use of your cosmetic ingredients is well established and known in the European cosmetic industry, the safety and efficacy should also be well understood, hence additional testing will not be required. However, if you want to introduce a new ingredient, then you must have evidence of its safety and efficacy before you can put it on the market.
Preparing a dossier for each ingredient is useful to organise all this data within your company. The ingredient dossier contains all the data for preparing the data sheets, as well as any marketing material. It is a resource for all published and proprietary data on the composition, the safety and efficacy of your ingredient, history of use, production methods and any other relevant information.
In this workbook, we will take you through the steps required to prepare the dossier for your ingredient. For such, we will use templates and other tools, including recommending a specific structure for the dossier itself.
2. Setting up your dossier
The ingredient dossier is the main company resource for all technical, economic, social, environmental data and other information about the ingredient.
The dossier must include the:
- the latest version of all the data sheets, as well as the older, redundant versions, which should be clearly marked;
- certificates of analysis;
- certificates of conformity.
The dossier also contains the ‘raw data’ from which all the data sheets were compiled. It is important for the dossier to be well structured, well organised and to remain updated. This will help you run your business more smoothly. It will save you time when responding to questions from European buyers and when you need to update the data sheets.
A suggested format for the dossier is presented below in Table 2, which can, of course, be adapted to the specific needs of your business.
Table 2: Typical contents of a technical dossier
| Structure of Dossier | |
| Basic documents | |
| TDS | Examples below |
| SDS | REACH format |
| Certificate of Analysis (CoA) | |
| Certificate of Conformity | Example below |
| PDS | |
| Product | |
| description | Plant part, etc. |
| origin | Wild or cultivated, area, country, region, etc. |
| Supply chain | |
| Community | |
| Photos | The plant, the community — consider the audience and the message in the photo |
| Traditional or historical use | Information about the traditional or historic use provides indication of safety, but history of use does not equate to safety. It also helps in the preparation of promotional material. Be sure to refer to already published information, unless you have the intellectual property rights or share these. |
| Processing | |
| Before receipt | |
| On site | |
| Process operations | |
| Safety data | Published by others and own data |
| Efficacy data | Published by others and own data |
| Technical analysis | Full composition |
| Physicochemical refer | |
| Toxicological | |
| Own analysis | |
| Published papers | What is relevant? Library description, refer to the modules. |
| Microbiological | |
| Pesticides | |
| Heavy metals | |
| Phthalates | |
| Certifications | |
| Access permits | Refer to access and benefit sharing legislation in your own country |
Tips:
- Some of the information in the dossier will be used to prepare public documents such as the TDS, the SDS and the PDS. See the chapter below.
- Other information that is not included in the TDS will occasionally be shared with customers, especially data that is not recorded for each batch, such as phthalates and heavy metals.
- Other information will be confidential and may not be shared at all with anyone outside the company, or only shared within the framework of a confidentiality agreement. There is no obligation to share any data even with a confidentiality agreement. Such information may include the type of processing used and proprietary data on efficacy.
3. Determining the INCI name for your ingredient
You can skip this step if your ingredient already has an INCI name.
INCI stands for ingredient nomenclature for cosmetic ingredients. All ingredients used in cosmetics need an INCI name. INCI names are based on the botanical names of plant, animal or microbial species, providing a harmonised approach to ingredient labelling and allowing for international recognition.
In the European Union, the glossary of common ingredient names used on product labels uses INCI names. New INCI names can also be used to help promote or launch a new ingredient. It is important to note that INCI registration is not a safety assessment. The INCI application and registration procedure is managed by the Personal Care Products Council (PCPC). If you have a new ingredient, then it is likely that no INCI name has been assigned yet. You will need to contact the PCPC and follow the INCI application procedure including payment of a relatively small fee.
PCPC publishes a dictionary of all INCI names. If you are a PCPC subscriber, you can access this dictionary. If you do not have access to the dictionary, you can contact your local cosmetic industry association to find out if they have access. In the European Union, you can find the registry of many INCI names in the CosIng database. However, CosIng is not a complete list of all known INCI names.
The INCI database is a form of market communication. Once the INCI name is published, then other companies with the same ingredient can use that INCI name too. Therefore, it is advisable to consider first completing all the necessary preparatory steps regarding safety in particular and efficacy, as well as preparing the technical data sheet and safety data sheet before applying for an INCI name. Then, once you have the INCI name, you are ready to start marketing your ingredient immediately.
Do you need to determine the INCI name for your ingredient? This flow chart can help you.
Type of content per product category and document type
Looking at the dossier in more detail, the following table summarises which data and information are required for each of the different categories of cosmetic ingredients. The tables also show where to use the information in the dossier to prepare the TDS, SDS and PDS documents. Examples of the TDS, CoA, SDS and PDS will be provided farther down in the guide.
The data gathering process typically begins with the data sheets and especially the TDS, based on the CoA data, followed by the SDS and PDS. In the table below, the crosses indicate which parameters need to be included in the data sheet. For example, the free fatty acid (FFA) levels are included in the TDS and CoA, but they are not needed in the PDS.
Table 3: Summary of requirements for vegetable oils
| Parameter | Dossier | TDS | CoA | SDS | PDS |
|---|---|---|---|---|---|
| Appearance | x | x | x | ||
| Full composition | x | ||||
| Moisture | x | x | x | ||
| Free fatty acids | x | x | x | ||
| Percentage of impurities | x | x | |||
| Specific gravity | x | x | x | x | |
| Fatty acid profile (generally not for each batch) | x | x | x | ||
| Microbiological contamination (normally not present) | x | x | x | ||
| Tocopherols and other antioxidants (periodic, not for each batch) | x | x | x | ||
| Phthalates (periodic, not for each batch) | x | x | x | ||
| Heavy metals (periodic, not for each batch) | x | x | x | ||
| Pesticide residues (periodic, not for each batch) | x | x | x | ||
| Protein content and allergen declaration | x | x | x | ||
| Iodine value | x | x | x | x | |
| Saponification value | x | x | x | x | |
| Peroxide value | x | x | x | ||
| Ash | x | ||||
| Unsaponifiables | x | ||||
| Refractive index | x | x | x | ||
| Ignition point | x | x | |||
| Rancimat stability (periodic) | x | x | x | ||
| Lovibond colour | x | x | x | ||
| Odour | x | x | x | ||
| Type and concentration of preservatives | x | x | |||
| Shelf life under stated conditions | x | x | |||
| Product name | x | x | x | x | x |
| Principle function in cosmetics | x | x | |||
| Components and characteristics | x | x | |||
| Botanical name | x | x | |||
| INCI name | x | x | x | x | x |
| CAS | x | x | x | x | x |
| EINECS | x | x | x | x | x |
| HS code | x | x | x | x | x |
| Product type (what form, liquid, powder, etc.) | x | x | |||
| Part of plant used | x | x | |||
| Cultivated or wild harvested | x | x | |||
| Access permits (ABS) | x | x | |||
| Plant description | x | x | |||
| Photos | x | x | |||
| Habitat | x | x | |||
| Distribution of plant | x | x | |||
| Location of raw materials | x | x | |||
| Medicinal uses of plant and plant parts | x | x | |||
| Toxic components (names, concentration) when applicable | x | x | x | x | |
| Traditional uses of plant and plant parts | x | x | |||
| Access permit and other certificates | x | x | x | ||
| Efficacy data | x | x | |||
| Efficacy data summary | x | x | |||
| Simplified description of manufacturing process | x | x | x | ||
| Manufacturing process | x | ||||
| Production volumes per year | x | ||||
| Purification stages | x | ||||
| Preservation steps | x | ||||
| Type of packaging | x | x | x | x | |
| Recommended storage | x | x | |||
| Published studies, reports, references on safety and efficacy | x | x | x | ||
| Toxicological tests | x | x | x | ||
| Local toxicity | x | x | x | ||
| Primary skin irritation | x | x | x | ||
| Ocular irritation | x | x | x | ||
| Allergenicity | x | x | x | ||
| Sensitisation | x | x | x | ||
| Systemic toxicity | x | x | x | ||
| Mutagenesis: Ames test | x | x | x | ||
| Acute toxicity | x | x | x | ||
| Efficacy studies (own, published) | x | x | x | ||
| Efficacy data summary | x | x | x | ||
| Summary of safety data | x | x | x | ||
| Recommended conditions of use: product type, part of body, frequency of use, method of application, concentration in cosmetic products | x | x | x | ||
| Environmental data | x | x | x | ||
| Socioeconomic information | x | x |
Table 4: Summary of requirements for essential oils
| Parameter | Dossier | TDS | CoA | SDS | PDS |
|---|---|---|---|---|---|
| GCMS composition (using gas chromatography-mass spectrometry) | x | x | x | ||
| Percentage of impurities | x | x | x | ||
| Moisture | x | x | x | ||
| Concentration levels of the 26 allergens (usually a separate declaration) | x | x | x | x | |
| Specific gravity | x | x | x | ||
| Flash point | x | x | x | x | |
| Optical rotation | x | x | |||
| Miscibility in ethanol | x | x | x | ||
| References to ISO standards | x | x | x | ||
| Organoleptic aspects | x | x | x | ||
| Shelf life under stated conditions | x | x | |||
| Product name | x | x | x | x | x |
| Description and aroma | x | x | x | x | x |
| Principal function in cosmetics | x | x | |||
| Key components | x | x | |||
| Botanical name | x | x | |||
| INCI name | x | x | x | x | x |
| CAS | x | x | x | x | x |
| EINECS | x | x | x | x | x |
| Part of plant used | x | x | |||
| Cultivated or wild harvested | x | x | |||
| Access permits (ABS) and other certifications | x | x | x | ||
| Plant description | x | x | |||
| Photos | x | x | |||
| Habitat | x | x | |||
| Distribution of plant | x | x | |||
| Location of raw materials | x | x | |||
| Medicinal uses of plant and plant parts | x | x | |||
| Toxic components (names, concentration) when applicable | x | x | x | x | |
| Pesticide residues (periodic, not for each batch) | x | x | x | ||
| Phthalates (periodic, not for each batch) | x | ||||
| Heavy metals (periodic, not for each batch) | x | ||||
| Simplified description of manufacturing process | x | x | x | ||
| Manufacturing process | x | ||||
| Production volumes per year | x | ||||
| Purification stages | x | ||||
| Preservation steps | x | ||||
| Type of packaging | x | x | |||
| Recommended storage | x | x | |||
| Published studies, reports, references on safety and efficacy | x | x | x | ||
| Toxicological test | x | x | x | ||
| Local toxicity | x | x | x | ||
| Primary skin irritation | x | x | x | ||
| Ocular irritation | x | x | x | ||
| Allergenicity | x | x | x | ||
| Sensitisation | x | x | x | ||
| Systemic toxicity | x | x | x | ||
| Mutagenesis: Ames test | x | x | x | ||
| Acute toxicity | x | x | x | ||
| Summary of safety data | x | x | x | ||
| Efficacy studies (own, published) | x | x | x | ||
| Efficacy data summary | x | x | x | ||
| Environmental data | x | x | x | ||
| Socioeconomic information | x | x |
Table 5: Summary of requirements for botanical extracts
| Parameter | Dossier | TDS | CoA | SDS | PDS |
|---|---|---|---|---|---|
| Full composition | x | ||||
| Key components in the plant extract | x | x | x | x | |
| Percentage of impurities | x | x | x | ||
| pH | x | x | x | x | |
| Refractive index | x | x | x | ||
| Solubility in water, oil, alcohol | x | x | |||
| Microbial analysis | x | x | x | ||
| Colour, odour | x | x | x | ||
| Moisture content | x | x | x | ||
| Concentration of alcohol | x | ||||
| Use of denatured alcohol? | x | ||||
| Shelf life under stated conditions | x | x | |||
| Product name | x | x | x | x | x |
| Principle function in cosmetics | x | x | |||
| Preservatives | x | x | x | ||
| Components and characteristics | x | x | x | ||
| Botanical name | x | x | |||
| INCI name | x | x | x | x | x |
| CAS | x | x | x | x | x |
| EINECS | x | x | x | x | x |
| Product type (liquid, powder, etc.) | x | x | |||
| Part of plant used | x | x | |||
| Cultivated or wild harvested | x | x | |||
| Access permits (ABS) and other certifications | x | x | x | ||
| Plant description | x | x | |||
| Photos | x | x | |||
| Habitat | x | x | |||
| Distribution of plant | x | x | |||
| Location of raw materials | x | x | |||
| Medicinal uses of plant and plant parts | x | x | |||
| Protein content and allergen declaration | x | x | x | ||
| Toxic components (names, concentration) when applicable | x | x | x | x | x |
| Traditional uses of plant and plant parts | x | x | x | x | |
| Efficacy data summary | x | x | x | x | |
| Efficacy data | x | x | |||
| Simplified description of manufacturing process | x | x | x | ||
| Description of manufacturing process | x | ||||
| Type of solvent | x | ||||
| Production capacity per year | x | ||||
| Purification stages | x | ||||
| Seasonality | x | ||||
| Steps to preserve – the critical steps | x | ||||
| Type of packaging | x | x | |||
| Storage conditions | x | x | x | ||
| Published studies, reports, references on safety and efficacy | x | x | x | ||
| Toxicological tests | x | x | x | ||
| Local toxicity | x | x | x | ||
| Primary skin irritation | x | x | x | ||
| Ocular irritation | x | x | x | ||
| Allergenicity | x | x | x | ||
| Sensitisation | x | x | x | ||
| Systemic toxicity | x | x | x | ||
| Mutagenesis: Ames test | x | x | x | ||
| Acute toxicity | x | x | x | ||
| Published studies, reports, references on safety and efficacy | x | x | x | ||
| Summary of safety data | x | x | x | ||
| Environmental data | x | x | x | ||
| Socioeconomic information | x | x | x |
Tips:
- Download the summary of requirements for essential oils.
- Download the summary of requirements for vegetable oils.
- Download the summary of requirements for botanical extracts.
- The dossier must be as complete as possible. The more data and information that you are able to prepare and collect, the greater the knowledge you will have about the ingredient and the more prepared you will be to deal with enquiries from European buyers.
- Keep each ingredient dossier well structured, well organised, well maintained and up to date, using clear indexes and sections. It is not just a repository, but a logically arranged reference document.
- If you have both electronic and paper records, ensure good cross-referencing as you compile the dossier.
- The dossier is not a static document, it must be regularly updated with your own company information and other published references.
- Be flexible. Some European buyers will require more data than others.
4. Preparing the Technical Data Sheet (TDS)
The technical data sheet (TDS) is a statement of the technical characteristics of your ingredient. It defines a particular quality that you, as a supplier, guarantees to supply. It also allows your buyers to check whether what they have purchased matches what they agreed to purchase. The TDS is also known as the Product Specification.
The structure of a typical TDS for the different categories of ingredients is shown in the sample below. When the raw data is available from your dossier, it will be a straightforward process to complete the TDS.
Sample TDS for vegetable oils
The following table contains notes about each of the sections in the specification for a vegetable oil.
Table 6: Notes for vegetable oils
| 1 | Product name (critical for the CoA) | The name of the product, which could be the trade name. |
| 2 | INCI name (critical for the CoA) | You must include this in the proper INCI format. See INCI nomenclature on the website of the Personal Care Product Council. |
| 3 | CAS | Add the CAS number if available. Check CosIng or the INCI dictionary. Not all ingredients have CAS numbers. The generic CAS number for vegetable oils is 68956-68-3. (All vegetable oils have the same basic triglyceride structure.) |
| 4 | European Inventory of Existing Commercial chemical Substances (EINECS) | Add the EINECS number if it exists. Check CosIng or the INCI Dictionary. |
| 5 | Description (critical for the CoA) | This is a basic description of the process used to make the extract. |
| 6 | Appearance (critical for the CoA) | This can be a visual assessment. For example, watery white, light yellow, orange, nearly colourless, dark green, golden yellow, yellow red. Colour can also be measured using a Lovibond meter. |
| 7 | Odour (critical for the CoA) | Typical odour of the product, for example: light, nutty, cocoa, green. |
| 8 | Moisture (critical for the CoA) | This should be zero, but less than 0.1% is acceptable. |
| 9 | Free fatty acids (critical for the CoA) | This should ideally be zero, but that is often not possible. Less than 2% is typical. Above that, there is a risk of rancidity and an unacceptably short shelf life. |
| 10 | Peroxide (critical for the CoA) | Indicator of quality. Should be less than 10 meq/kg. |
| 11 | Saponification value (critical for the CoA) | A type of fingerprint test, along with iodine value and fatty acid profile. The saponification values for oils are well known and therefore an anomalous result may indicate adulteration or a different oil. |
| 12 | Iodine value (critical for the CoA) | A type of fingerprint test, along with saponification value and fatty acid profile. |
| 13 | Specific gravity (critical for the CoA) | A measure of quality. An abnormal specific gravity (SG) reading may suggest adulteration with other fluids. |
| 14 | Fatty acid profile (critical for the CoA) | The TDS should include the typical fatty acid composition range, ideally accounting for 100% of all the fatty acids in the oil. Some buyers expect to have a fatty acid analysis for every batch. Others will accept an analysis on a regular basis, such as twice a year. |
| 15 | Microbiological analysis (critical for the CoA) | This entails total plate count, coliforms, moulds and yeasts. This should be zero in the absence of moisture. It is a standard test. Discuss with the microbiological laboratory. |
| 16 | Use of preservatives | Only add this parameter if preservatives are used. Indicate the concentration level and type of preservative. Try to avoid use of preservatives. Recommend flushing the headspace in the containers with nitrogen. |
| 17 | Shelf life and storage | Indicate a total number of months in which the specification is maintained. Indicate under which conditions the shelf life is valid. Mention that the headspace has been flushed with nitrogen, if this has been done. |
| 18 | Packaging | Indicate how the customer could receive the product, for example, in containers of 25 kg up to 200-litre drums or larger. |
| 19 | HS Code | Use this code to help buyers calculate tariffs, if applicable. Use the EU Trade Helpdesk to identify HS codes, discuss with your freight forwarder or customs agency. |
| 20 | Certifications and permits | Include details of certifications and biodiversity access permits where applicable. |
Tips:
- Download a template TDS for vegetable oils.
- Download an example of TDS for organic virgin coconut oil.
- Download an example of TDS for cocoa butter.
- Download an example of TDS for organic sunflower oil.
- The TDS should be based on the cumulative experience of analysing batches. A TDS should be based on the actual analysis of at least three batches. The more batches used the more accurate the TDS data. In most circumstances, it is better to offer a single quality of the identical ingredient rather than too many variants. Hence blending batches to achieve a harmonised TDS is often carried out. Traceability of the batches is very important.
- Some buyers will agree to some tests being carried out on a periodic basis. Other buyers will expect a full Certificate of Analysis for every batch. These are matters for discussion and negotiation with the buyer. The more assurance you can give of quality consistency in your batches and the greater attention you give to traceability and quality assurance along the supply chain, the more flexible they can be. This is also related to availability of documentation showing clear policies and procedures to ensure quality at every step of the supply chain.
- For physical and chemical testing, you can use laboratories that have ISO17025 certification.
- For toxicology tests, you should use laboratories that have Good Laboratory Practice (GLP) accreditation.
- The laboratory that you use should have access to the details of the common methods of analysis for the parameters of the specification. In the case of vegetable oils, the Federation of Oils, Seeds and Fats Associations (FOSFA) has published a list of contractual methods of analysis as a reference.
- It is important to record the methods used to carry out the analysis of each of the parameters. These methods should be recorded in the dossier where they can be easily accessed in case of any questions from buyers, especially in the case of discrepancies between the results obtained by a buyer and your results.
- On our website, you can find a number of factsheets about with information on exporting natural ingredients for cosmetics to Europe.
- It is best not to publish your TDS on your website. You can publish your PDS. Once a buyer has contacted you, you can send them the TDS directly.
- A very useful reference for anyone who is supplying vegetable oils for the cosmetic industry is the safety assessment of plant-derived fatty acid oils published by the Cosmetic Ingredient Review (no login required). This report contains sample specifications for a wide range of vegetable oils, as well as information on their safety in use in cosmetics. The report is well referenced and is itself used as a reference for typical specifications of vegetable oils.
Sample TDS for essential oils
Find below information on developing a TDS specifically for essential oils, including making a separate allergen declaration. The following table contains notes on each of the sections in the specification for essential oils.
Table 7: Notes for essential oils
| 1 | Product name (critical for the CoA) | The name of the product, which could be the trade name. |
| 2 | INCI name (critical for the CoA) | You must include this in the proper INCI format. See INCI nomenclature on website of Personal Care Product Council. |
| 3 | CAS | Add the CAS number if you can find it. Check CosIng or the INCI dictionary. |
| 4 | EINECS | Add the EINECS number if it exists. Check CosIng or the INCI Dictionary. |
| 5 | Description and aroma (critical for the CoA) | This is the basic description of the process, e.g. steam distillation. Aroma is the most important parameter, followed by composition. |
| 6 | Composition including allergen declaration (as a separate document) (critical for the CoA) | This is the primary reference. Include the most important components in the essential oil based on long-term experience of the results of the GCMS analysis. |
| 7 | Allergen declaration (critical for the CoA) | Attach as separate document reference to the allergen composition (26 allergens). See the tips below. |
| 8 | Optical rotation (critical for the CoA) | Identification parameter |
| 9 | Specific gravity (critical for the CoA) | Identification parameter |
| 10 | Flash point (critical for the CoA) | Used to assess transport hazard. Please see the SDS for more information. |
| 11 | Packaging | Indicate how your buyer could receive the product. |
| 12 | Shelf life and storage | State the shelf life under given conditions of storage. |
| 13 | HS code | Use this code to help the buyer calculate the tariffs if applicable. |
| 14 | Certifications and permits | Include details of certifications and biodiversity access permits where applicable. |
Tips:
- Download a template TDS for essential oils.
- Download an example of TDS for rosemary oil.
- Download an example of TDS for geranium oil.
- On our website you can find a number of factsheets about specific essential oils.
- The International Standards Organisation (ISO) has published a number of standards for essential oils, including analytical methods, which provide good industry benchmarks for quality.
- A useful reference for essential oil is the International Fragrance Association (IFRA), which publishes industry codes of conduct and standards for use of fragrances and fragrance components.
- Check an example of an allergen declaration for clove oil and an example of an allergen declaration for lavender oil.
Sample TDS for botanical extracts
The table below gives an overview of the sections to include in the certificate of analysis (see chapter 5) for botanical extracts. This is also buyer dependent.
Table 8: Notes for botanical extracts
| 1 | Product name (critical for the CoA) | The name of the product, which could be the trade name. |
| 2 | INCI name (critical for the CoA) | You must include this in the proper INCI format. See INCI nomenclature on website of Personal Care Product Council. |
| 3 | CAS | Include the CAS number if you can find it. Check CosIng or the INCI dictionary. The CAS number for a naturally occurring substance, for instance, plant extract is 999999-99-4. |
| 4 | EINECS |
Add the EINECS number if it exists. Check CosIng or the INCI Dictionary. |
| 5 | Description (critical for the CoA) | This is a basic description of the process used to make the extract. |
| 6 | Key components in the plant extract | Include here any standardised components such as anti-oxidants etc. Also include the maximum concentration of any toxic components. Also include percentage of alcohol, if applicable. |
| 7 | Appearance | Colour, viscosity if liquid, or powder. |
| 8 | Odour | Typical odour profile. |
| 9 | pH | In the case of aqueous products |
| 10 | Miscibility in water, oil, alcohol (critical for the CoA) | To understand the physical characteristics |
| 11 | Moisture content (critical for the CoA) | Indicator of stability, especially for powders, for example, maximum 8% moisture. |
| 12 | Microbiological analysis (critical for the CoA) | Especially if the product contains water |
| 13 | Use of preservatives (critical for the CoA) | Only add this parameter if preservatives are used. Indicate the concentration level and type of preservative. Try to avoid using preservatives. If unavoidable, use food preservatives such as sodium benzoate. Certain pH levels help control microbial spoilage. |
| 14 | Storage and shelf life (critical for the CoA) | State the shelf life under certain conditions of storage. |
| 15 | Packaging | Indicate how the customer could receive the product. |
| 16 | HS code | Use this code to help the buyer calculate the tariffs if applicable. |
| 17 | Certifications and permits | Include details of certifications and biodiversity access permits where applicable. |
Tips:
5. Preparing the Certificate of Analysis (CoA)
The Certificate of Analysis is used to check whether a batch conforms to the specifications detailed in your TDS. Your buyers may carry out their own analyses of key parameters to check conformance with the TDS.
The parameters in a CoA are the key parameters to be analysed for every batch. You may not always need to test for all the same parameters in the TDS. However, this can also depend on your customer.
6. Preparing the Certificate of Conformity (CoC)
The Certificate of Conformity is a document authorised by the person responsible for quality in the company. This certificate is a signed statement that the Certificate of Analysis for a particular batch of product conforms with the TDS or Specification. Your buyers may also carry out their own analyses of key parameters to check conformance with the TDS.
Tip:
7. Preparing the Safety Data Sheet (SDS)
A Safety Data Sheet (SDS) is a key document in the overall safety of the supply, handling and use of chemicals. It should help to ensure that those who use chemicals in the workplace do so safely without risk to users or the environment.
European Union legislation on safety data sheets is the regulation on Registration, Evaluation, Authorisation and Registration of Chemicals (REACH) EC 1907/2006, as amended by 453/2010. For the cosmetic industry, all ingredients require a safety data sheet, whether they are classified as dangerous or not. This includes samples, ingredients and chemicals that are exempt from specific chemical registration requirements.
Vegetable oils that are not chemically modified, for example, are exempt from the chemical registration legislation. But for transportation purposes and for sale to a European manufacturer, a vegetable oil requires an SDS.
The development of an SDS requires detailed technical knowledge about your product. The person responsible for preparation of the SDS must understand the implications of their statements in the SDS.
The European Chemicals Agency (ECHA), which oversees the implementation of the REACH legislation, has many guidelines and tools advising on the preparation of safety data sheets. It is recommended that you and the person responsible for the preparation of the SDS study this information if you are not familiar with SDS preparation.
This workbook uses questions to take you through the 16 sections of an SDS. By answering the questions, you develop the most essential information for an SDS. This basic SDS will provide the critical information for safe trade of your product.
The following flowchart is included in the ECHA’s Guidance on the Compilation of Safety Data Sheets, describing the recommended process of collecting and collating the data. However, you will see that the numbering system in the boxes is not sequential. The numbers refer to the order within the SDS, not the order of collection and collation of the data.
Figure 1: SDS compilation flowchart

The SDS must comply with the requirements of the EU REACH Regulation 1907/2006; Annex II as amended by EC 2015/830.
The SDS must follow a 16-section format as follows:
Section 1: Identification of the substance or mixture and of the company or undertaking
Section 2: Hazards identification
Section 3: Composition and information on ingredients
Section 4: First aid measures
Section 5: Firefighting measures
Section 6: Accidental release measures
Section 7: Handling and storage
Section 8: Exposure controls and personal protection
Section 9: Physical and chemical properties
Section 10: Stability and reactivity
Section 11: Toxicological information
Section 12: Ecological information
Section 13: Disposal considerations
Section 14: Transport information
Section 15: Regulatory information
Section 16: Other information
You can use the Classification and Labelling Inventory of the European Chemicals Agency (ECHA) to help you answer the questions below. This database, however, contains only information on registered substances for which other companies have already provided safety data to the ECHA.
An example of an SDS for cardamom oil is provided in the right column of the table below.
Use the following tables to answer all relevant questions for every step of the SDS:
Section 1 Identification of the substance or mixture and of the company or undertaking | Examples of answers for cardamom oil |
Section 1.1 What is the trade name for your product? What is the CAS number? What is the EINECS number? | Section 1.1 Trade name: Cardamom oil CAS number: 8000-66-6 EINECS number: 288-922-1 |
Section 1.2 What are the details of the supplier of this SDS? | Section 1.2 Your company name and address |
Section 1.3 What is the emergency telephone number of the supplier of this SDS? | Section 1.3 Your emergency telephone number |
| SECTION 2: Hazards identification | Examples of answers for cardamom oil | |||
Section 2.1 What are the hazard classifications of the product? What hazard statements apply to your product?
Classification: Flammable liquid 3 Skin irritant 2 Skin sensitizer 1 Aquatic chronic 2 | Section 2.1
Hazard statements: H226: Flammable liquid and vapour H315: Causes skin reaction H317: May cause an allergic skin reaction H411: Toxic to aquatic life with long lasting effects | |||
Section 2.2 Which signal word has to be used on the label for your product? Signal word: | Section 2.2
| |||
GHS02
| GHS07
| GHS09
| ||
Hazard determining substance: Precautionary statements: | Cardamom oil P210: Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. P241: Use explosion-proof electrical, ventilating, lighting, equipment. P261: Avoid breathing dust, fume, gas, mist, vapours and spray. P303+P361+P353: if on skin or hair, take off immediately all contaminated clothing. Rinse skin with water or shower. P321: Specific treatment (see on this label). P501: Dispose of contents and container in accordance with local, regional, national and international regulations. | |||
| SECTION 3: Composition/information on ingredients | Examples of answers for cardamom oil |
Section 3.1 What is the chemical characterisation, for example, the substance for essential oils? What is the CAS number description? What are the identification numbers, such as EINECS, REACH registration and FEMA codes? | Section 3.1 Chemical characterisation: substance
CAS number description: 8000-66-6 Cardamom oil EINECS number: 288-922-1
|
| SECTION 4: First aid measures | Examples of answers for cardamom oil |
Section 4.1 What first aid measures need to be taken following inhalation, skin contact, eye contact or ingestion? | Section 4.1 Description of first aid measures: Immediately remove any clothing soiled by the product. After inhalation: Supply fresh air and, to be sure, see a doctor. In case of unconsciousness, place patient stably in side position for transportation. After skin contact: Immediately wash with water and soap and rinse thoroughly. After eye contact: Rinse opened eye for several minutes under running water. After swallowing: If symptoms persist, consult doctor. |
| SECTION 5: Firefighting measures | Example for cardamom oil |
Section 5.1 What extinguishing agents should be used in case of fire? Section 5.2 Special hazards arising from substance or mixture Section 5.3 What is the advice for firefighters? | Section 5.1 Section 5.2 On combustion emits toxic fumes of carbon monoxide, carbon dioxide and unidentified organic compounds. Section 5.3 Advice for firefighters: No special measures required.
|
| SECTION 6: Accidental release measures | Example for cardamom oil |
Section 6.2 What environmental precautions need to be taken?
Section 6.3 What measures need to be taken for cleaning up?
| Section 6.2 Environmental precautions: Do not allow product to reach sewage system or any water course. Inform respective authorities in case of seepage into water course or sewage system. Do not allow to enter sewers, surface or ground water.
Section 6.3 Measures for cleaning up: Absorb with liquid-binding material such as sand, diatomite, acid binders, universal binders, sawdust. Dispose contaminated material as waste according to item 13. Ensure adequate ventilation.
|
SECTION 7: Handling and storage
| Example for cardamom oil |
Section 7.1 What precautions for safe handling need to be taken?
Section 7.2 What measures need to be taken for fire and explosion protection?
Section 7.3 What are the requirements for storage rooms?
What are the requirements for receptacles?
What is the recommended storage temperature? | Section 7.1 Precautions for safe handling: Ensure good ventilation and exhaust at the workplace. Prevent formation of aerosols.
Section 7.2 Measures for fire and explosion protection: Keep ignition sources away. Do not smoke. Protect against electrostatic charges.
Section 7.3 Requirements for storage rooms: No special requirements. Requirements for receptacles: Keep container tightly sealed. Cool, well-ventilated area, away from direct sunlight. |
| SECTION 8: Exposure controls and personal protection | Example for cardamom oil |
Section 8.1 What are the control parameters for safety monitoring? Section 8.2 What protective and hygienic measures must be taken?
What respiratory protection must be taken?
What eye protection must be taken? What body protection must be taken?
| Section 8.1 Control parameters: No monitoring required.
Section 8.2 Protective and hygienic measures: Keep away from foodstuffs, beverages and feed. Immediately remove all soiled and contaminated clothing. Wash hands before breaks and at the end of work. Avoid contact with the skin. Avoid contact with the eyes and skin. Respiratory protection: In case of brief exposure or low pollution, use respiratory filter device. In case of intensive or longer exposure, use self-contained respiratory protective device. Eye protection: Tightly sealed goggles. Body protection: Protective gloves. The glove material has to be impermeable and resistant to the product, substance or preparation. |
| Section 9: Physical and technical properties | Examples of answers for cardamom oil |
Use results of the laboratory analysis to answer the following questions: Section 9.1
Section 9.2
| Section 9.1
Section 9.2
|
| SECTION 10: Stability and reactivity | Example for cardamom oil |
Section 10.1 What is the thermal decomposition of the product? What hazardous reactions are possible? | Section 10.1 Thermal decomposition of the product: No decomposition if used according to specifications. Dangerous reactions: No dangerous reactions known.
|
| SECTION 11: Toxicological information | Example for cardamom oil |
If toxicological information is not available in the ECHA database, check eChemPortal or TOXNET for toxicity information for your product.
Section 11.1 What are the main toxic effects?
|
Section 11.1 Main toxic effects: Causes skin irritation. May cause an allergic skin reaction. Based on available data, the classification criteria for other toxic effects are not met. |
SECTION 12: Ecological information
| Example for cardamom oil |
If ecological information is not available in the ECHA database, check eChemPortal or TOXNET for this information for your product. Section 12.1 What are the main toxic effects on the environment? |
Section 12.1 Main toxic effects on the environment: Toxic for fish and other aquatic organisms. Danger to drinking water if even small quantities leak into the ground |
| SECTION 13: Disposal considerations | Example of answer for cardamom oil |
Check the list of waste established by European Union Regulation 2000/532 for disposal considerations. What is the recommended waste treatment method, for example, incineration, recycling or landfill? Discourage sewage disposal. |
Recommended waste treatment method: Do not allow product to reach ground water, water course or sewage system. Must not be disposed together with household garbage.
|
| SECTION 14: Transport information | Example for cardamom oil |
Provide transport information by mode of transport: • Land: International Carriage of Dangerous Goods by Road (ADR) • Marine: International Maritime Dangerous Goods (IMDG) • Air: Safe Transport of Dangerous Goods by Air (IATA)
Section 14.1 What are the UN numbers for the substances in the product, divided by mode of transport?
Section 14.2 What is the proper shipping name, divided by mode of transport?
Section 14.3 What is the transport hazard class, divided by mode of transport?
Section 14.4 What is the packing group, divided by mode of transport? Section 14.5 What are the environmental hazards, for example, marine pollutant?
Section 14.6 What special precautions need to be taken by the user? | Section 14.1 UN number: 1169 Sections 14.2-14.6 ADR
Section 14.2 UN proper shipping name: EXTRACTS, AROMATIC, LIQUID, ENVIRONMENTALLY HAZARDOUS
Section 14.3 Class: 3 (Flammable liquids)
Section 14.4 Packing group: III Environmental hazard: Toxic to aquatic life with long lasting effects. Special precautions: • Danger code (Kemler): 30 • EMS Number: F-E, S-D • Stowage category: A • Limited quantities: 5L • Excepted quantities: Code: E1 • Maximum net quantity per inner packaging: 30 ml • Maximum net quantity per outer packaging: 1000 ml Transport category: 3 Tunnel restriction code: D/E IMDG, IATA UN proper shipping name: EXTRACTS, AROMATIC, LIQUID Class: 3 (Flammable liquids) Packing group: III
Section 14.5 Environmental hazard: Toxic to aquatic life with long lasting effects.
Section 14.6 Special precautions: • Danger code (Kemler): 30 • EMS Number: F-E, S-D • Stowage category: A • Limited quantities: 5L • Excepted quantities: Code: E1 • Maximum net quantity per inner packaging: 30 ml • Maximum net quantity per outer packaging: 1000 ml |
SECTION 15: Regulatory information
| Example of answers for cardamom oil |
Section 15.1 What specific European safety, health, and environmental regulations apply to your product, for example, Regulation 850/2004 on persistent organic pollutants?
What specific national regulations apply to your product in your country?
Section 15.2 Have you carried out a Chemical Safety Assessment?
| Section 15.1 European safety, health and environmental regulations:
Section 15.2 A Chemical Safety Assessment has not been carried out. |
| SECTION 16: Other information | Example of answer for cardamom oil |
What are the full meanings of the acronyms and abbreviations used in this SDS?
What key literature has been used for compilation of this SDS? | Acronyms and abbreviations:
Key literature: This information is based on our present knowledge. However, this shall not constitute a guarantee for any specific product features and shall not establish a legally valid contractual relationship.
|
See three actual examples of SDSs for cardamom oil from the following companies:
Tips:
- Download a blank template of an SDS. Please note that the template is only a guide. It will need to be completed by a specialist for your particular ingredient, whether it is a substance or a mixture.
- Download an example of SDS for a vegetable oil (baobab oil).
- Download an example of SDS for an essential oil (chamomile essential oil).
- Download an example of SDS for a plant extract (pomegranate glycerol extract).
- SDSs are not confidential. However, simply copying one from the internet for your ingredient is not recommended. Perhaps an existing SDS can be used as a guide, but ultimately, you are responsible for the compilation of your SDS as a supplier, therefore you must be sure of the data you are including in your SDS. Simply copying off the internet without knowing what you are doing is a mistake. In case of doubt contact an agency that can advise you.
- When you do not have the data for specific sections in your SDS, you must state ‘No Data Available’. In some cases, especially for ingredients that are known to be safe, the data is not needed. However, in other cases where ingredients are dangerous, such information is legally required for safe handling and use. In these cases, it is advisable to talk to your buyer or your freight forwarder.
- Do you have a registration number for the SDS? Registration numbers are awarded to European Union companies or Only Representatives that have registered their imported or manufactured substances or mixtures with ECHA. Such companies include the registration number in their own SDS, in compliance with European Union law. Without registration of chemicals that fall within the scope of the legislation, the importer or European manufacturer cannot sell those chemicals in the European Union. It is not obligatory for an exporter from outside the EU to register with REACH. There are a number of possible statements that could be used in this section instead of the exporters to the European Union registration number, although it is not legally required to make any statement. See page 33 of ECHA’s Guidance on the Compilation of SDS for more information.
- If you are new to the SDS documentation for cosmetic ingredients, do take the time to read the Guidance on the Compilation of Safety Data Sheets. It is written in clear English and is comprehensive.
- See our study on REACH for more information about it. You can also use ECHA’s Guidance on REACH and the ECHA Guidance in a Nutshell .
- ECHA has also published a very useful SDS checklist. It is designed from an inspector’s point of view but the aim is to improve the quality of safety data sheets in the European Union. It is useful to compare your SDS to the standard detailed in this checklist. It is also available in some other European languages besides English.
- There are many resources on the internet that can assist with preparing an SDS. Another SDS template can be found online here.
- Are you not sure whether you have the technical knowledge in your company to develop an SDS? Contracting an accredited laboratory to develop an SDS for your product is always recommended.
- Are you certain that you have the technical knowledge? Then you can use this workbook to prepare a basic SDS with the most essential information yourself.
8. Preparing the Product Data Sheet (PDS)
The PDS is a marketing tool that combines selected technical information about the product combined with information about the:
- supply chain;
- collection or cultivation of the source materials;
- processors or collectors involved, where this may be of interest to a buyer;
- traditional uses, especially cosmetics or food.
The PDS could also include information about local beauty rituals and traditions of use, where relevant. The PDS may also include photos and references from published documents.
Most suppliers present their PDSs on their corporate websites. It is the primary and first communication vehicle for your ingredient. Technical data sheets are often not published in full on company websites, as they might disclose too much information and help competitors. It is common to present a summary of the key attributes and benefits.
Tips:
- Review as many companies as possible to see how they present their product data sheets. There are many ways to do this. You can visit websites to view examples or ask for a PDS at a trade fair.
- Have a look at the PDS for a range of products presented by Lipoid Kosmetik or see the PDS for a range of vegetable oils presented by IMCD.
9. Sampling procedures
European buyers normally do not request samples when they first contact suppliers, especially in the case of ingredients that are not used in their fragrances. There is usually a long period where documents are exchanged, including the exchange of confidentiality agreements, before they request a sample.
Buyers of fragrance ingredients can have a different approach. Fragrance is such an important aspect of cosmetics that often fragrance is a primary deciding factor. After a sample is approved then a trial order may be placed.
How much you should send as a sample depends on:
- which type of ingredient;
- why it is requested;
- how it is used in a cosmetic;
- how much it costs.
Typically, you might be asked to send:
- 50 ml of a vegetable oil;
- 10 ml of an essential oil,
- 1–50 g of a botanical extract, depending on the concentration.
Expect your buyer or prospective customer to pay for the sample; however, there is no guarantee that they will pay. It depends on the buyer and it depends on the ingredient. When discussing samples, ask for their international courier account number, if they have one.
Shipping small quantities of dangerous chemicals is very expensive. For that reason, exhibiting at a trade fair is an excellent way to hand over samples of essential oils, for example.
Tips:
- Keep track of all the documents and certificates of analysis during the sampling procedure. Ensure that all samples are batch numbered and traceable.
- Keep samples of all batches in case of any questions about the quality of a shipment. Cosmetic ingredients can be perishable. However, usually the quality remains consistent for the few months between purchase and use in manufacturing.
- If you work with European distributors, make sure they have enough samples to send to their customers.
10. Corporate Social Responsibility
Compliance with the principles of corporate social responsibility (CSR) is nowadays a compulsory element of preparation for the international market. Buyers want to ensure their suppliers are compliant with international laws, the laws and regulations where they operate as well as whether vendors are committed to practices meeting their own CSR practices.
Companies are expected to have documents outlining their policies relating to labour standards, health and safety standards, ethical business standards and environmental standards. Without this documentation buyers may lose confidence in you and see you as untrustworthy. Many companies are now going beyond their legal requirements and using standards in their supply chains like Fair Trade and the Union for Ethical Bio Trade (UEBT).
We can consider two broad aspects of corporate social responsibility. The first is the adherence to standards of CSR within the exporter company, meaning the elements of social responsibility that are under direct control of the owners of the company. The second aspect is the social responsibility within the supply chain. Of course, expecting your suppliers to adopt the same standards of social responsibility as yours, as their customer, is a logical expectation, especially in the eyes of your customers and their customers.
At the same time, it is possible to incorporate other additional aspects of social responsibility within your supply chain that go beyond the conventional aspects of corporate responsibility, such as Fair Trade and Biodiversity Sustainability.
Company level
SEDEX
Many buyers in Europe and around the world subscribe to SEDEX. In our surveys of European companies all have said that working with suppliers who also subscribe to SEDEX is an advantage.
SEDEX is a platform that allows you to share information about your responsible sourcing business with your buyers and potential buyers. It involves completing a self-assessment questionnaire and adding audit reports and certifications. SEDEX does not offer a set standard, it is independent from any single standard or methodology. There is a membership fee of approximately £100 per year if you are a supplier. There are other subscriptions for buyers and other companies further downstream. Using SEDEX will also help suppliers prepare for most CSR questions from buyers, for example, in buyer questionnaires, as mentioned below.
The SEDEX self-assessment questionnaire cover topics in the following four categories:
- Labour
- Health and Safety
- Business Ethics
- Environment
Figure 2: SEDEX self-assessment questionnaire topics, accessible with SEDEX membership:
For each category there are series of questions. See Figure 3 below, for example, for the category of Labour.
Figure 3: Example Labour category questions from the SEDEX self-assessment questionnaire:
Figure 4: Example extract from SEDEX self-assessment questionnaire:
It is also possible to upload external audits and other documents. The SEDEX self-assessment questionnaire is available in 15 languages.
If a buyer who also subscribes to SEDEX is interested in seeing your SEDEX profile, the buyer will request your SEDEX reference number. Apart from the profile, your information is confidential and not available outside the SEDEX system. More detailed information about SEDEX is available from the SEDEX website and in the SEDEX workbook.
Supply chain
The standard aspects of corporate social responsibility in your supply chain are equally important to your suppliers. Your customers expect not to find child labour, for example, at your factory nor anywhere in your supply chain. Therefore, applying the SEDEX questionnaire to your own suppliers is a very good practice.
Traceability
Traceability has always been important, but has taken on a new level of significance under the European cosmetics regulation. Increased interest in certification is driven by the need for traceability. Certification with external standards, whether organic, natural, fair trade, fair wild or biodiversity, provide traceability in addition to the requirements of the particular standard. In particular, when companies invest so much in promoting specific ingredients from specific sources, they need to have complete confidence in the origin.
External standards
Some buyers require external standards as well as a company’s own documentation on CSR. External standards require a third party to independently verify CSR policies and often have fees associated with them. External standards provide traceability and transparency along the whole supply chain. Some standards, such as Fair Trade and organic certification, require every link in the supply chain to be certified if a company wishes to use their logo. These companies will not source their ingredients from you if you do not have this certification.
Fair Trade
Fair trade certifications are offered by a number of different accreditation services. Common across all certifications for fair trade is ensuring that producers receive fair prices and a focus on social, economic and environmental development for the producers. The definition of fair trade differs depending on the fair-trade certifier. The following certifying bodies offer fair trade certification: Ecocert/IMO, Control Union and Fairtrade International.
Biodiversity: UEBT Standard
The Union for Ethical Bio Trade (UEBT) standard focuses on sourcing with respect to people and biodiversity in the natural ingredients for sectors such as cosmetics, pharmaceuticals and food. The UEBT standard is built on BioTrade principles and criteria developed by the UNCTAD BioTrade initiative.
Topics of the UEBT Standard
Figure 5: Topics of the UEBT Standard

Natural and Organic standards
Cosmos
Cosmos is a standard applied to cosmetic products that are marketed as natural or organic. The standard covers all aspects of sourcing, manufacture, marketing and control of cosmetic products. Products that are certified by Cosmos must be formulated using only ingredients that the standard allows. Raw materials must either be certified or approved — certified ingredients have organic content, approved raw materials are non-organic.
To certify your ingredients with organic content you need to apply to an authorised certification body, audited before you receive your certification. To gain approval for your non-organic content ingredients, you must apply to an authorised body which will send you a raw materials questionnaire to be verified before granting approval. Once certified or approved your ingredients will appear on the databases for certified ingredients or approved raw materials.
NATRUE
NATRUE is a not-for-profit association based in Belgium that has developed standards for natural cosmetics, natural cosmetics with an organic portion and organic cosmetics. NATRUE has classified ingredients used in cosmetics into three basic types: natural, derived natural and nature identical ingredients. Nothing artificial or man-made is allowed in NATRUE certified cosmetics. A manufacturing company that aims to obtain NATRUE certification for its products can refer to the list of compliant ingredients on the NATURE raw materials database. Suppliers of raw materials seeking to obtain NATRUE certification can contact NATRUE for information on how to register ingredients on this database.
Organic
Organic certification involves a set of production standards for growing, storing, processing, packaging and shipping that generally avoids synthetic chemical inputs, irradiation, genetically modified seed, farmland that has received chemical inputs within a certain amount of time. Organic certification also generally requires keeping detailed records on production and sales, strict physical separation of organic products from non-certified products and undergoing periodic on-site inspections. In Europe, where organic is a legally defined term, labelling food organic and importing organic products are strictly controlled.
Organic products imported from non-European countries can only be distributed in the European market if they are produced and inspected under conditions that are identical or equivalent to the standards of European producers. You can obtain organic certification from a number of different accrediting bodies. European organic legislation is published under the following:
Council Regulation (EC) No 834/2007
There is no specific legislation for organic cosmetics. The private standards developed by COSMOS and NATRUE, for example, use organic food legislation as a reference for an organic quality in cosmetics.
IS0 26000
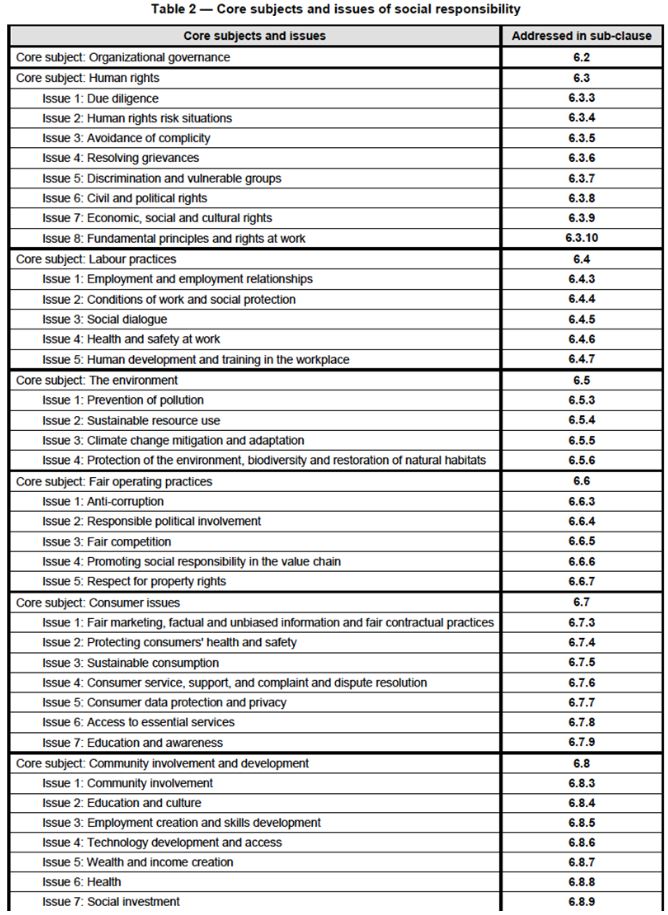
ISO 26000:2010 is intended to assist organisations in contributing to sustainable development. It cannot be certified like other ISO standards. This standard provides guidance on the underlying principles of social responsibility, the core subjects of social responsibility, recognising social responsibility as an organisation, engaging stakeholders and integrating social responsibility into an organisation.
Figure 6: The core subjects and issues of social responsibility
United Nations Global Compact
The United Nations Global Compact is a global corporate sustainability initiative that supports companies to do business responsibly and take strategic action to develop broader societal goals, such as the United Nations Sustainable Development Goals. The Global Compact has ten principles based around human rights, labour, the environment and anti-corruption. Companies can become signatories or participants in the Compact by preparing a Letter of Commitment. The Letter of Commitment contains: a statement expressing support for the compact, a description of the practical actions that the company has taken or plans to take to implement the ten principles of the compact and a measurement of the outcomes, i.e. the degree to which the targets or performance indicators were met. To be a signatory, companies must pay an annual contribution depending on annual sales revenues. Companies with average sales revenues under 25 million US dollars are asked to contact their Local Network to discover if a contribution applies. To be a participant, annual contribution starts at 1,250 US dollars for companies with annual sales revenue under 25 million US dollars.
The UN Global Compact is not a performance or assessment tool. It does not provide a seal of approval, nor does it make judgments on performance. Participants are expected to publish in their annual report or similar corporate report (e.g. sustainability report) a description of the ways in which they are supporting the UN Global Compact and its ten principles. This is known as the Communication on Progress. The UN Global Compact believes that this sort of openness and transparency encourages improved practices by participants.
The Communication on Progress (COP) is frequently the most visible expression of a participant's commitment to the Global Compact and its principles. According to the policy, failure to issue a COP will change a participant’s status to non-communicating and can eventually lead to the expulsion of the participant. You can find out more about how to participate in the Global Compact here.
The United Nations Sustainable Development Goals
Adopted by all United Nations Member States in 2015, the 2030 Agenda for Sustainable Development provides a shared blueprint for peace and prosperity for people and the planet, now and into the future. At its heart are the 17 Sustainable Development Goals (SDGs), which are an urgent call for action by all countries in a global partnership. They recognize that ending poverty and other deprivations must go hand in hand with strategies that improve health and education, reduce inequality and spur economic growth while tackling climate change and working to preserve the world’s oceans and forests.
The SDGs result from an inclusive process that has governments involving businesses, civil society and individuals. Fulfilling these ambitions will take an unprecedented effort by all sectors in society. Business has an important role in the process.
Companies are encouraged to do their part in achieving the SDGs under the commitment towards corporate social responsibility in many ways. Click on this link for more information about how companies can contribute to the Sustainable Development Goals.
Figure 7: The United Nations Sustainable Development Goals

Company Questionnaires
The majority of all companies send supplier questionnaires to all new and potential suppliers. Please find below an example of one such questionnaire. In many cases, using SEDEX will prepare you to answer many of the questions that buyers ask.
These questionnaires usually request the following information about your company. At the end of it, a declaration must be signed by a senior representative of the company.
Company details
- Quality
- Regulatory
- Manufacturing
- Health and Safety
- Social Responsibility
- Environment
- Declaration
EXAMPLE BASIC SELF-AUDIT FORM
Please complete in full and return this form to the requested
- COMPANY DETAILS
-
- Company Name and Address
|
-
- Manufacturing site(s) and address
If more than one address, please attach a list of all sites specifying products made at each location
|
-
- Is production of any of your materials outsourced to another manufacturer?
If yes, please provide information
o Yes
o No
|
-
-
- Production capacity of each product to be supplied (in tonnes)
-
……………tonnes
|
1.4 Business type
o Private owned
o Public limited
o State owned
|
1.5 Please confirm your role in the supply chain
o Manufacturer
o Agent
o Distributor
o Other, namely:
|
1.6 Year business commenced
Business commenced in: ……………
|
1.7 Please provide a copy of your company organogram
Copy attached:
o Yes
o No (proprietary information)
|
1.8 Key personnel contacts
Please provide name, job title, contact number and email address
Sales
|
Name: Job title: Contact number: Email address:
|
Quality
|
Name: Job title: Contact number: Email address:
|
Regulatory
|
Name: Job title: Contact number: Email address:
|
Technical
|
Name: Job title: Contact number: Email address:
|
1.9 Emergency 24-hour contact: name, company name and contact number
Emergency contact details
|
Name: Company name: Contact number:
|
1.10 Do you have current sales in Europe or do you work with a distributor in Europe?
If yes, please provide details
|
- QUALITY
-
- Are you ISO-9001 certified?
Please attach certificate and contents page of your Quality Management System, then proceed to section 3. If no, please complete section 2
o Yes
o No
|
-
- Do you have a documented Quality Management System? If yes, how often is it reviewed?
Please include a copy of the index page or Standard Operating Procedure (SOP) and outline content of SOP
|
2.3 What is the format and frequency of internal audits?
If referring to a SOP, please provide a copy or outline of content of SOP
|
2.4 What, if any, quality management tools are being used to continuously improve site performance?
If referring to a SOP, please provide a copy or outline of content of SOP
|
2.5 What procedures are in place for dealing with customer complaints?
If referring to a SOP, please provide a copy or outline of content of SOP
|
2.6 How is complaint and feedback information used to improve processes and product quality?
If referring to a SOP, please provide a copy or outline of content of SOP
|
2.7 How often are customer and internal complaint trends reviewed?
If referring to a SOP, please provide a copy or outline of content of SOP
|
- REGULATORY
3.1 How do you keep up to date and compliant with applicable EU legislation?
|
3.2 Has your company received any regulatory actions in the last three years?
If yes, please provide details and list corrective actions you have taken
|
3.3 Please attach a European SDS/MSDS, typical specification and a CoA for a product.
Documents attached:
o Yes
o No
|
3.4 If you are able to provide SDSs/MSDSs in other European languages, please list those that are available.
|
3.5 Please provide information or a status on the REACH status of your company and products.
|
3.6 How are products tested and verified to ensure compliance with applicable regulatory requirements and
performance standards?
If referring to a SOP, please provide a copy or outline of content of SOP
|
- SITE AND MANUFACTURING
-
- How do you approve your raw material suppliers?
If referring to a SOP, please provide a copy or outline of content of SOP
|
4.2 Do you hold specifications agreed with your suppliers for all raw materials purchased?
o Yes
o No
|
4.3 Do you have a procedure to check if incoming raw materials meet specifications?
If yes, please provide information. If referring to a SOP, please provide a copy or outline of SOP
o Yes
o No
|
4.4 Are handwashing facilities located at each entrance to production and packaging areas?
o Yes
o No
|
4.5 What checks and procedures are in place to ensure that all machinery and tools are in good condition, adequately maintained and fit for purpose?
If referring to a SOP, please provide a copy or outline of SOP
|
4.6 Are all critical items of plant equipment subject to scheduled and traceable calibration?
If referring to a SOP, please provide a copy or outline of SOP
|
4.7 Do you use an in-house or contract laboratory to test products? If yes, are they formally accredited, for example, by UKAS, CLAD or LabCred?
If yes, please provide information
In-house o Contract laboratory o
|
Yes o No o
|
4.8 Do you quality control check each batch to specification?
If yes, please provide information. If referring to a SOP, please provide a copy or outline of SOP
o Yes
o No
|
4.9 Do production lines include:
|
4.10 Do you have systems in place to retain samples of all product batches and to verify the shelf life of products?
If yes, please provide information. If referring to a SOP, please provide a copy or outline of SOP
|
4.11 Do you have any control procedures in place for the following:
4.11.1 Allergens
If yes, please provide information
o Yes
o No
|
4.11.2 Cross contamination
If yes, please provide information
o Yes
o No
|
4.11.3 Foreign bodies
If yes, please provide information
o Yes
o No
|
4.11.4 Stock segregation
If yes, please provide information
o Yes
o No
|
4.12 What checks and controls are in place to ensure finished products are adequately packaged and correctly labelled at the point of dispatch?
If referring to a SOP, please provide a copy or outline of SOP
|
4.13 What is the cleaning schedule for:
4.13.1 Warehouse
|
4.13.2 Manufacturing area
|
4.13.3 Packing area
|
4.14 Do you have a formal recall procedure?
If yes, please provide information. If referring to a SOP, please provide a copy or outline of SOP
o Yes
o No
|
4.15 Describe contingency plans in case of an event which prevents manufacture of products and could affect supply.
|
- HEALTH AND SAFETY
5.1 Is there a communicated and measurable health and safety policy in place?
If yes, please provide information. If referring to a SOP, please provide a copy or outline of SOP
|
5.2 Do you have formal risk assessments such as HACCP systems in place?
Hazard analysis and critical control points, or HACCP, is a systematic approach to food safety and
pharmaceutical safety that identifies physical, allergenic, chemical and biological hazards in production processes that can cause the finished product to be unsafe, and designs measurements to reduce these risks to a safe level.
Please also provide a flowchart
o Yes
o No
|
If yes to the questions 5.1 and 5.2, please continue to section 6. If no, please complete the remaining questions in this section
5.3 What is the procedure for reporting accidents that occur on site?
If referring to a SOP, please provide a copy or outline of SOP
|
5.4 What procedures and controls are in place to manage major accidents, emergencies or breaches of legislation?
If referring to a SOP, please provide a copy or outline of SOP
|
5.5 What first-aid and medical facilities are available on site?
|
5.6 Are fire extinguishers frequently serviced, readily available? Have workers been trained in their use?
|
5.7 How often are fire evacuation drills conducted and what details are recorded?
|
5.8 How is the wearing of personal protective equipment (PPE) controlled and enforced on site?
|
5.9 Is the site covered with a pest control contract?
Please provide details on level of cover and attach contract
|
- ETHICS
6.1 Are you registered with SEDEX?
SEDEX is a not-for-profit membership organization dedicated to driving improvements in ethical and responsible business practices in global supply chains.
If yes, please provide registration number
o Yes, registration number: ………………………………………….
o No
|
6.2 Do you have a corporate social responsibility policy prohibiting the use of:
6.2.1 Child labour
o Yes
o No
|
6.2.2 Slavery
o Yes
o No
|
6.2.3 Payment or acceptance of bribes or inducements
o Yes
o No
|
6.3 Do workers have freedom of association?
o Yes
o No
|
6.4 Do you have SA8000 certification?
Please attach certificate
o Yes
o No
|
6.5 Do you have training systems and records of all employee training?
If yes, please provide information. If referring to a SOP, please provide a copy or outline of SOP
- ENVIRONMENT
-
- Do you hold an ISO-14100 certification?
If yes, please attach certificate and policy, then proceed to signature page. If no, please complete questions 7.2 and 7.3
o Yes
o No
|
7.2 Is there a communicated and measurable environmental policy in place?
If yes, please provide information
o Yes
o No
|
7.3 What are the company’s environmental objectives?
|
I hereby confirm that all information provided is accurate and agree to immediately notify you in writing of any change to the above details:
Authorised signature: _________________________________________
Name: ______________________________________________________
Position: ____________________________________________________
Email: ______________________________________________________
Telephone number: ___________________________________________
Date: _______________________________________________________
Please review our market information disclaimer.
Search
Enter search terms to find market research


